We are excited to share our new preprint on bioRxiv, Functionally Essential and Structurally Diverse: Insights into the Zebrafish Left-Right Organizer’s Cilia via Optogenetic IFT88 Perturbation and Volume Electron Microscopy . This work was led by Favour Ononiwu (Hehnly lab Graduate student) with contributions from Melissa Mikolaj (NCI- Narayan Lab), Christopher Dell (NCI-Narayan Lab), Abdalla Wael Shamil (Hehnly lab undergraduate), Kedar Narayan (NCI), and Heidi Hehnly.
Why this study matters
During vertebrate embryogenesis, the left-right organizer (LRO) generates asymmetric fluid flow that initiates left-right body patterning. In zebrafish, this role is carried out by a transient epithelial organ known as Kupffer’s Vesicle (KV). The cilia within KV have long been known to generate flow, but their structural heterogeneity and contribution to KV morphogenesis have remained unclear.
Our approach
We combined two powerful tools:
Optogenetics: Using a newly engineered sox17:Cry2-GFP zebrafish line, we clustered the intraflagellar transport protein IFT88 in KV progenitors via blue-light activation. This perturbation impaired ciliogenesis and disrupted lumen formation, establishing a direct role for cilia in KV morphogenesis.
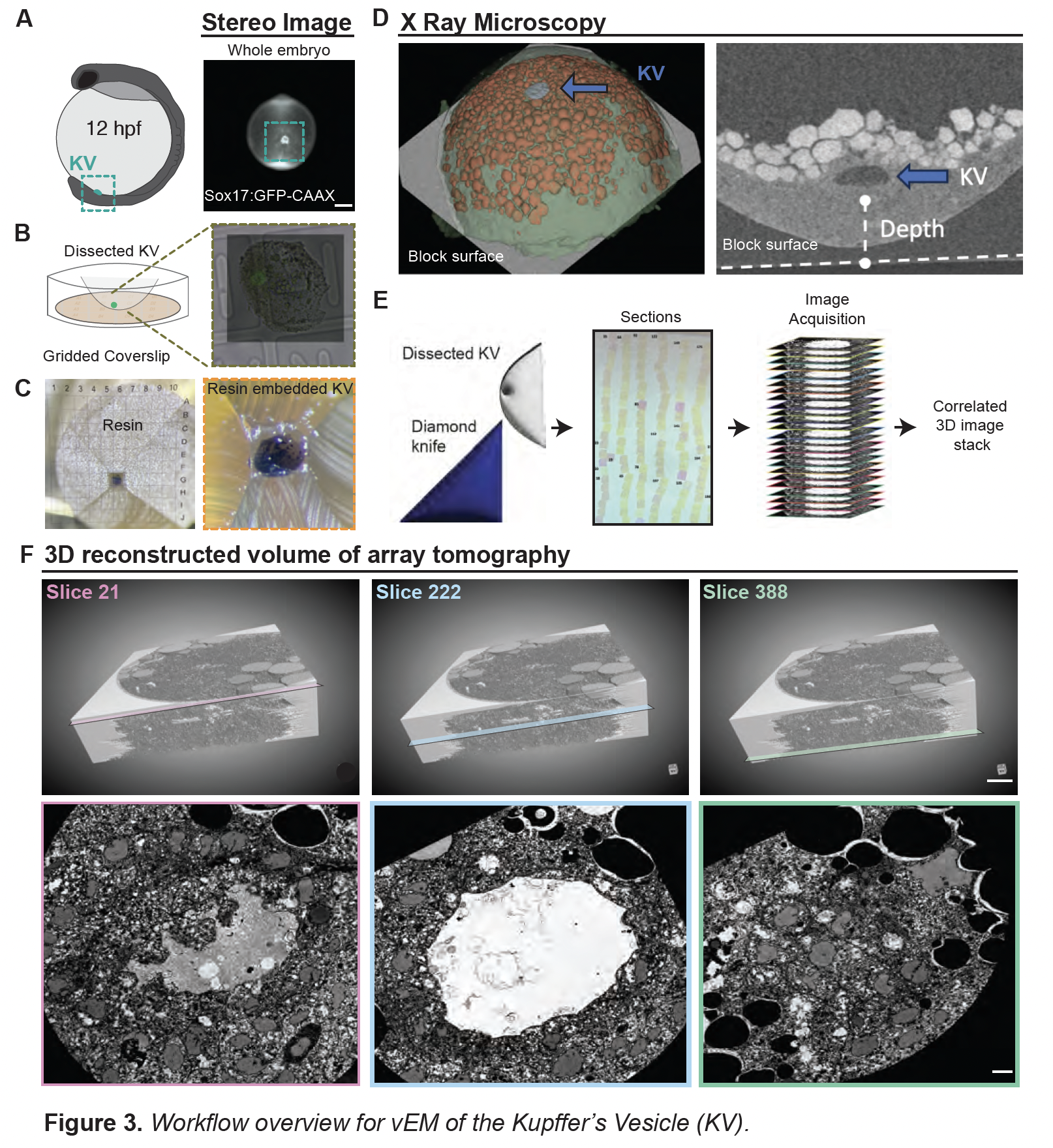
Volume electron microscopy (vEM): We generated the first high-resolution 3D ultrastructural map of a mature KV, enabling unprecedented analysis of ciliary architecture across the tissue.
Key findings
Only ~70% of cilia retained both mother and daughter centrioles, suggesting that centriole elimination may occur during KV development.
Among centrioles, distal appendages (34%), subdistal appendages (92%), and rootlet fibers (5%) were present in highly variable patterns, revealing remarkable structural diversity.
Cilia were frequently associated with membrane-bound vesicles, including ciliary-associated vesicles (CaVs) and dense vesicles (CaDVs), with distinct spatial distributions across the KV.
Broader implications
Our findings uncover previously unrecognized complexity in LRO organization. The structural specialization of KV cilia suggests that they may contribute not only to generating flow but also to organizing the architecture of the organ itself. This work adds to a growing appreciation that cilia are not uniform organelles, but instead exhibit context-specific diversity that underpins their function.
Next steps
We anticipate that these insights will inform broader studies of ciliary specialization across tissues and their role in developmental disorders linked to left-right asymmetry.