Under stress, certain eukaryotic proteins and RNA assemble to form membraneless organelles known as stress granules. The most well-studied stress granule components are RNA-binding proteins that undergo liquid-liquid phase separation (LLPS) into protein-rich droplets mediated by intrinsically disordered low-complexity domains (LCDs). Here we show that stress granules include proteasomal shuttle factor UBQLN2, an LCD-containing protein structurally and functionally distinct from RNA-binding proteins. In vitro, UBQLN2 exhibits LLPS at physiological conditions. Deletion studies correlate oligomerization with UBQLN2’s ability to phase-separate and form stress-induced cytoplasmic puncta in cells. Using nuclear magnetic resonance (NMR) spectroscopy, we mapped weak, multivalent interactions that promote UBQLN2 oligomerization and LLPS. Ubiquitin or polyubiquitin binding, obligatory for UBQLN2’s biological functions, eliminates UBQLN2 LLPS, thus serving as a switch between droplet and disperse phases. We postulate that UBQLN2 LLPS enables its recruitment to stress granules, where its interactions with ubiquitinated substrates reverse LLPS to enable shuttling of clients out of stress granules.
Hehnly Lab has a paper at Molecular Biology of the Cell!
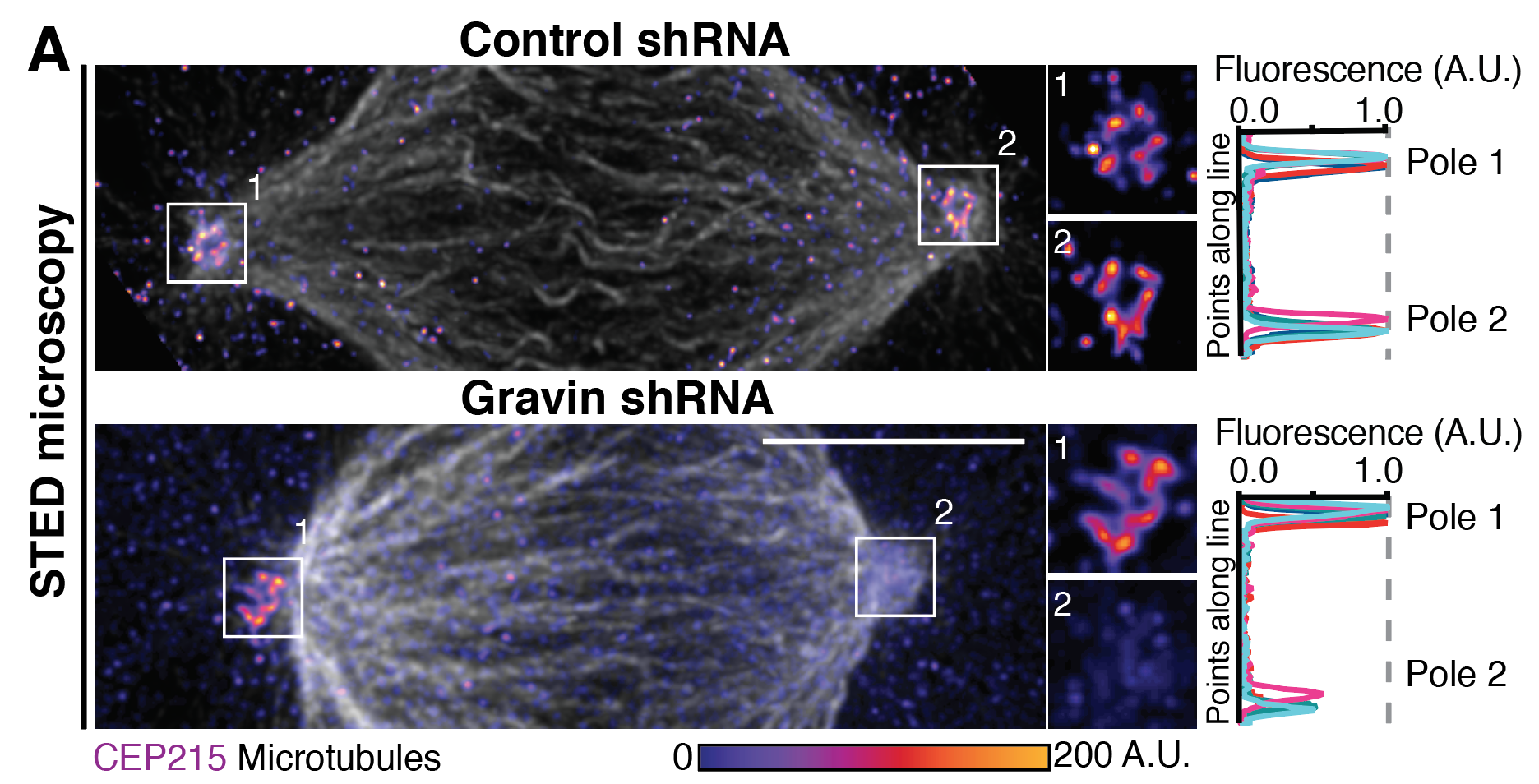
Colicino et al. talks about the role of Gravin anchoring PLK1 at mitotic centrosomes. This anchor is important for regulating centrosome organization and function as seen below with super resolution imaging of the centrosome component CEP215 disorganization when Gravin is lost.
A panel taken from Figure 4 from Colicino et al. of CEP215 disorganization primarily at a single mitotic spindle pole with Gravin loss. We argue this is through uncontrolled phosphorylation of CEP215 by PLK1 in the absence of Gravin.
Look at the Beautiful Paper!
The Hehnly Lab recently got to help with a story from Dylan Burnette's laboratory that came out in Scientific Reports. Check it out here. It is filled with beautiful microscopy and looks at the relationship between how a dividing cell communicates with an extracellular matrix, and its affect on cleavage furrow morphology, and spindle orientation.