We are excited to share our new collaborative paper with the Manning and Amack labs, published in PNAS. This work addresses a fundamental question in developmental biology: how do cells and tissues achieve the precise shapes required for organ function?
Why this matters
Many studies have focused on how cell-intrinsic properties—like signaling pathways or cytoskeletal dynamics—contribute to tissue shape. But development is more than just cells behaving individually; it is also about how tissues as a whole generate and respond to forces. Recent theoretical work suggests that embryonic tissues exist near a “jamming” transition, meaning they can flow very slowly but still transmit large forces over long timescales. These dynamic forces, though often overlooked, have been hypothesized by Manning and Amack groups to play a powerful role in shaping organs.
Our focus: Kupffer’s vesicle
To test this idea, Amack and Manning labs turned to Kupffer’s vesicle (KV), a transient, ciliated organ in zebrafish embryos that our lab also loves to examine. KV plays a crucial role in establishing left-right asymmetry during development, making it an ideal model to study how tissues generate and respond to mechanical forces.
What we did
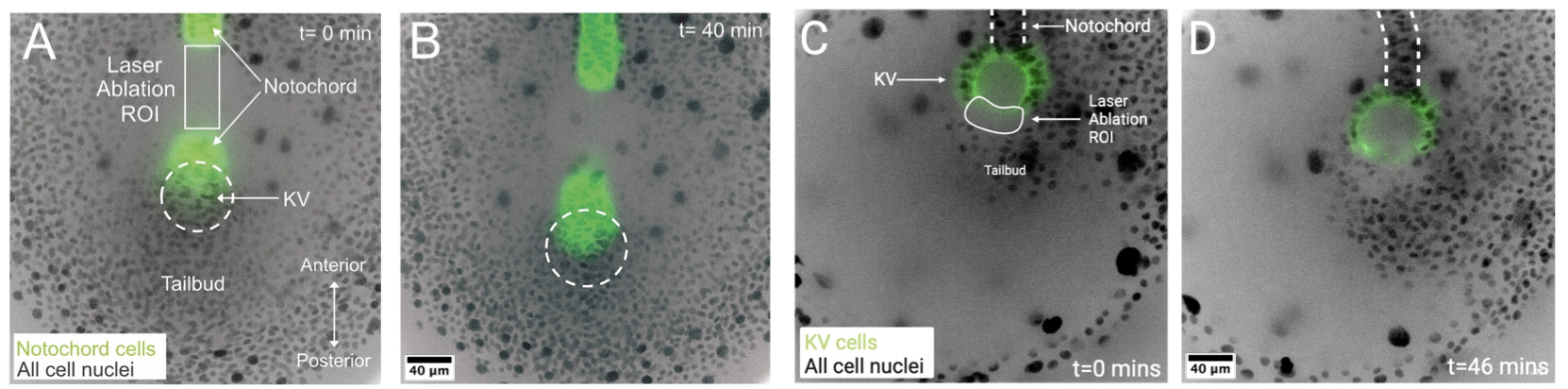
This project combined mathematical modeling, live imaging, and in vivo perturbations to test whether dynamic forces generated by tissue movements sculpt KV shape. The last part with in vivo perturbations, is where our group played an important role first with Mike Bates (a postbac in our lab and then Manager of the Blatt Imaging Center) and then with Yiling Lan (a graduate student in the lab).
Modeling predicted that slow tissue flows during embryogenesis could apply significant stresses to KV, driving its morphological changes.
Laser ablation experiments, performed in our lab (by Mike and Lan), were critical to test these predictions. By precisely severing tissue connections in the embryo, we altered force transmission and directly observed the resulting effects on KV shape. The outcomes matched the model predictions, providing strong evidence that dynamic forces are a key driver of organ morphology.
The bigger picture
The collaborative findings show that self-generated dynamic forces sculpt organ shape during development. Because many developmental processes occur on slow timescales, this principle likely applies broadly beyond zebrafish KV. This work opens the door to exploring how tissues harness dynamic mechanical forces across diverse developmental contexts.
We are thrilled to have contributed to this collaborative effort—particularly by performing the ablation experiments—and to see how interdisciplinary approaches combining modeling, physics, and cell biology can shed new light on fundamental developmental mechanisms.